FDA Issues Updated Final Guidance on Cybersecurity in Medical Devices
FDA issues final guidance on cybersecurity in medical devices, outlining QMS, premarket documentation, and Section 524B requirements for manufacturers.
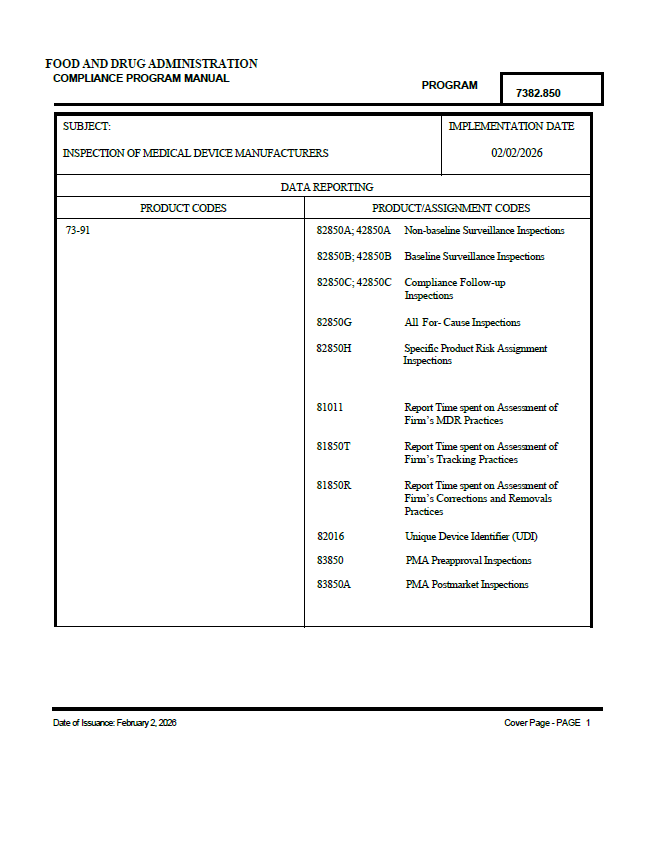
FDA Implements Updated Medical Device Inspection Compliance Program
FDA implements Compliance Program 7382.850, aligning medical device inspections with the Quality Management System Regulation and ISO 13485.
FDA Publishes Updated Guidance on Clinical Decision Support Software
The FDA has issued updated guidance on Clinical Decision Support (CDS) software, clarifying which software functions are excluded from the medical device definition under U.S. law.
New FDA Draft Guidance Sets Bar for Cuffless Blood Pressure Devices – Clinical Performance Now in Focus
The FDA has released a draft guidance outlining new clinical performance requirements for cuffless non-invasive blood pressure devices. Manufacturers using wearable sensors or AI must now meet specific testing standards, including ISO 81060-2. Learn how these updates affect premarket submissions and regulatory strategies.
FDA Releases Draft Guidance on Bayesian Methodology in Clinical Trials – What It Means for Innovative Study Designs
FDA releases draft guidance on Bayesian methods in clinical trials. Learn how device manufacturers can benefit from this innovative approach.
FDA Updates Guidance on Clinical Decision Support (CDS) Software for Healthcare Professionals
New FDA guidance clarifies which Clinical Decision Support software functions are not considered medical devices. Key updates affect software developers and manufacturers.
New FDA Guidance Clarifies Policy for Low-Risk General Wellness Devices
The FDA has updated its guidance on low-risk general wellness devices, clarifying criteria for software, wearables, and lifestyle technologies that may fall outside device regulation. Effective January 6, 2026.
FDA Finalizes Guidance on Sponsor Responsibilities for Safety Reporting in IND and BA/BE Studies
The FDA has finalized its 2025 guidance for sponsors on safety reporting in IND and BA/BE studies. Learn what manufacturers must update and how it affects your trials.
FDA Publishes Final Guidance on Computer Software Assurance: Key Implications for Manufacturers
The FDA has released its final guidance on Computer Software Assurance (CSA) for production and quality system software, introducing a risk-based, streamlined approach to validating software used in manufacturing and quality operations. The updated framework helps manufacturers reduce unnecessary documentation, focus validation efforts on functions that impact product quality and patient safety, and accelerate the adoption of automated and digital technologies while remaining fully compliant with FDA requirements.
FDA Issues Updated Scientific Recommendations for Biosimilars: What Manufacturers Need to Know
The FDA has issued a new draft guidance that updates the scientific principles for demonstrating biosimilarity. The document clarifies when strong analytical similarity data, supported by comparative human pharmacokinetic and immunogenicity assessments, may be sufficient to demonstrate biosimilarity without requiring a comparative clinical efficacy study. This updated approach can help manufacturers reduce development timelines and costs, while placing greater emphasis on robust analytical packages, sensitive PK study designs and a clear, risk-based justification aligned with FDA expectations.
FDA Draft Guidance Aligns QMS Requirements with ISO 13485: What It Means for Medical Device Manufacturers
The FDA has released a draft guidance aligning U.S. Quality Management System (QMS) requirements with ISO 13485:2016. This change simplifies compliance for medical device and IVD manufacturers and distributors, ensuring global harmonization and streamlined FDA premarket submissions effective February 2026.
FDA Issues Strategic Guidance on Decentralized Clinical Trials – A Turning Point for Medical Device and IVD Manufacturers
The latest FDA guidance on decentralized clinical trials (DCTs) marks a significant development for IVD and medical device manufacturers. By incorporating digital health technologies, remote monitoring, and home-based device usage, DCTs allow for enhanced trial flexibility and patient accessibility. This guidance clarifies regulatory expectations, especially around investigational product distribution, informed consent, and oversight, supporting innovation while maintaining compliance.
FDA publica novas especificações técnicas para submissão de dados clínicos em ensaios sobre leucemias agudas
A FDA lançou novas especificações técnicas para submissão de dados clínicos em leucemias agudas, definindo padrões rigorosos de qualidade e interoperabilidade.
FDA publica orientação sobre Computer Software Assurance para sistemas de produção e qualidade
A FDA publicou a orientação final sobre Garantia de Software Informático (CSA) para software de produção e sistemas de qualidade. Esta abordagem baseada no risco ajuda os fabricantes a garantir a conformidade, concentrar os esforços de validação onde são mais necessários e adotar tecnologias inovadoras, como automação, IA e sistemas em nuvem — melhorando, em última análise, a qualidade dos dispositivos médicos e a segurança dos pacientes.
FDA publica orientação sobre políticas de aplicação para testes de diagnóstico in vitro durante emergências de saúde pública
A FDA publicou uma nova orientação sobre políticas de enforcement aplicáveis a testes de diagnóstico in vitro durante emergências de saúde pública, ao abrigo da secção 564 do FD&C Act. O guia define os critérios para o início e término destas políticas, oferecendo previsibilidade aos fabricantes em situações críticas, sem comprometer a segurança pública.
FDA publica versão final da orientação ICH E6(R3) sobre Boas Práticas Clínicas
A FDA publicou a versão final do guia ICH E6(R3) sobre Boas Práticas Clínicas (GCP), que substitui a versão anterior e estabelece novos requisitos para ensaios clínicos. O documento reforça a abordagem baseada no risco, a integração tecnológica e a proteção dos participantes, promovendo qualidade, ética e harmonização internacional nos dados clínicos submetidos a processos regulamentares.
FDA atualiza orientações sobre qualificação de Pequenas Empresas no âmbito do MDUFA: Oportunidade estratégica para fabricantes
A FDA publicou uma nova orientação para a qualificação como pequena empresa no âmbito do MDUFA, permitindo a fabricantes de dispositivos médicos e IVDs acederem a reduções nas taxas regulamentares, incluindo submissões 510(k), PMA e De Novo.
FDA reforça a cibersegurança na produção de dispositivos médicos: novas orientações para fabricantes
A nova orientação da FDA sobre cibersegurança na produção de dispositivos médicos alerta para os riscos associados à tecnologia operacional (OT) utilizada em ambientes industriais. A FDA recomenda o mapeamento das redes conectadas, a implementação de arquiteturas seguras e a integração de normas como IEC 62443 e NIST SP 800-82.
FDA publica uma proposta de orientação sobre requisitos de UDI para produtos combinados
O novo guia preliminar da FDA sobre UDI em produtos combinados descreve requisitos detalhados para a rotulagem, identificação e submissão ao GUDID. Esta orientação afeta fabricantes de dispositivos médicos e medicamentos combinados, que deverão avaliar se os seus produtos requerem UDI, NDC ou ambos, conforme o tipo de componente principal.
FDA publica nova orientação sobre cibersegurança em dispositivos médicos – o que os fabricantes devem saber
A nova orientação da FDA sobre cibersegurança em dispositivos médicos define como os fabricantes devem incorporar práticas de segurança digital desde o design até à submissão pré-mercado. A abordagem inclui modelação de ameaças, utilização de um Secure Product Development Framework (SPDF) e a apresentação de um Software Bill of Materials (SBOM).