Swissmedic Publishes Updated EUDAMED UDI Enumerations: What Manufacturers Should Know
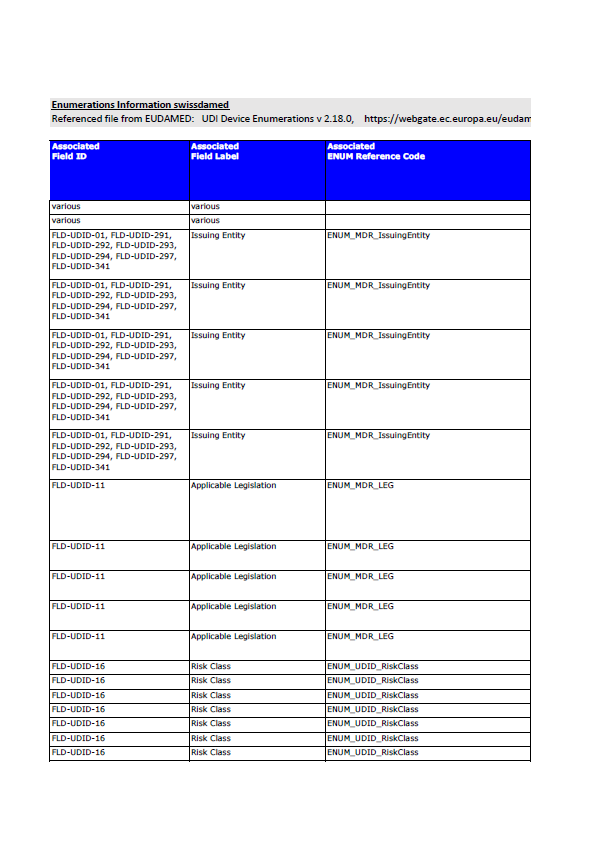
Swissmedic publishes updated EUDAMED UDI enumerations (v2.18.0). What this means for manufacturers preparing UDI, device and certificate data submissions.
Swissmedic Updates eIAM Portal Administrator Guidance – Version 3.4 Effective February 2026
Swissmedic publishes eIAM Portal Administrator guidance version 3.4, effective February 2026. Learn what this means for manufacturers using Swiss eGov services.
FDA Issues Updated Final Guidance on Cybersecurity in Medical Devices
FDA issues final guidance on cybersecurity in medical devices, outlining QMS, premarket documentation, and Section 524B requirements for manufacturers.
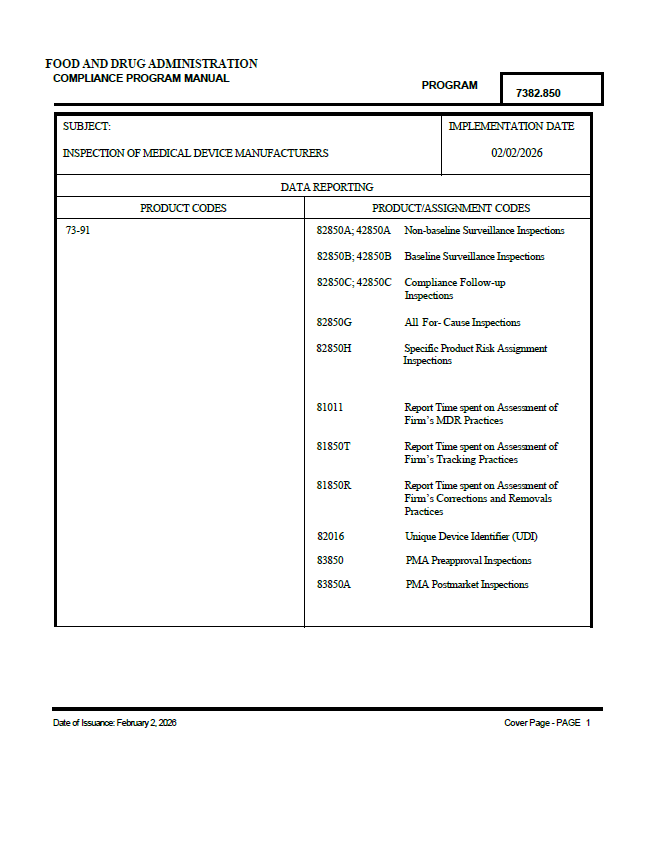
FDA Implements Updated Medical Device Inspection Compliance Program
FDA implements Compliance Program 7382.850, aligning medical device inspections with the Quality Management System Regulation and ISO 13485.
European Commission Publishes New Harmonised Standards for Sterilisation and IVD Labelling under IVDR
The European Commission has adopted Commission Implementing Decision (EU) 2026/197 of 28 January 2026, amending Implementing Decision (EU) 2021/1195 by adding new references to harmonised standards supporting Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR). The Decision was published in the Official Journal of the European Union on 30 January 2026 and entered into force on the same day.
EU-U.S. Data Privacy Framework Updated: What It Means for Medical Device Manufacturers
New EU-U.S. Data Privacy Framework FAQ impacts data transfers to U.S. vendors. What this means for medical device manufacturers in 2026.
MedTech Europe Proposes Targeted Reforms to MDR and IVDR – Strategic Input for 2026 Regulatory Revisions
MedTech Europe’s MDR/IVDR reform proposals remain key as the EU prepares its 2026 legislative update. Find out what this means for manufacturers.
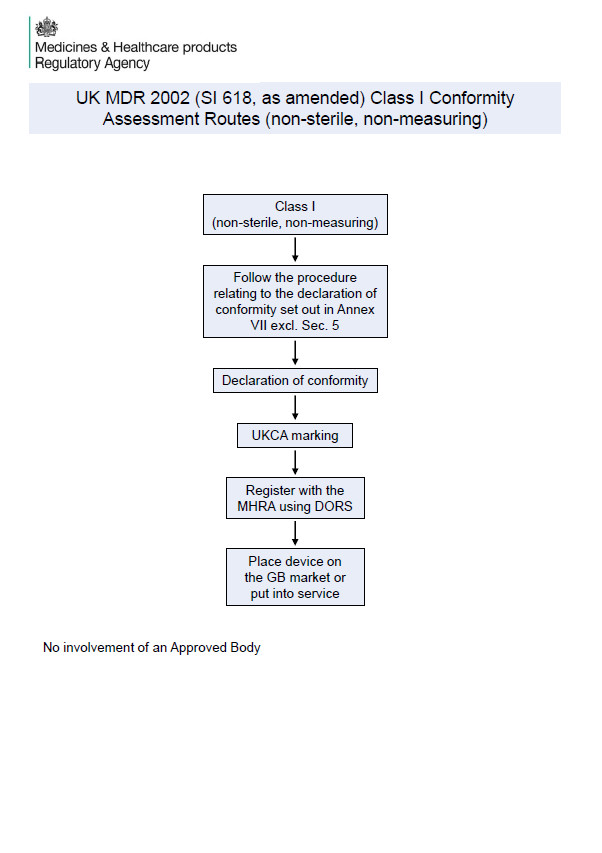
New MHRA Summary: UK Conformity Assessment Routes for All Device Classes
New MHRA guidance summarises UK conformity routes for all medical device classes. Essential for UKCA compliance and UK MDR planning.
FDA Publishes Updated Guidance on Clinical Decision Support Software
The FDA has issued updated guidance on Clinical Decision Support (CDS) software, clarifying which software functions are excluded from the medical device definition under U.S. law.
Australia Updates Guidance on Export Certificates for Medical Devices: What EU Manufacturers Need to Know
The TGA has updated its guidance on Certificates of Free Sale and Export Certificates for medical devices. Key changes for EU manufacturers exporting via Australia.
Swissmedic Highlights Critical Deficiencies in Notified Body Opinions for Integral Combination Products
Swissmedic’s review of Notified Body Opinions under MDR Article 117 reveals major gaps in documentation quality for drug–device combination products. Updated expectations now apply to ensure compliance with GSPRs.
Swissmedic Updates swissdamed Business Rules – Key Changes for Device Manufacturers in 2026
Swissmedic has released version 3.0 of its swissdamed Business Rules, effective January 2026. The update includes stricter UDI-DI requirements, limits on legacy device data, and mandatory fields for MDR/IVDR submissions. Manufacturers must now align with Swiss-specific data rules diverging from EUDAMED.
New FDA Draft Guidance Sets Bar for Cuffless Blood Pressure Devices – Clinical Performance Now in Focus
The FDA has released a draft guidance outlining new clinical performance requirements for cuffless non-invasive blood pressure devices. Manufacturers using wearable sensors or AI must now meet specific testing standards, including ISO 81060-2. Learn how these updates affect premarket submissions and regulatory strategies.
FDA Releases Draft Guidance on Bayesian Methodology in Clinical Trials – What It Means for Innovative Study Designs
FDA releases draft guidance on Bayesian methods in clinical trials. Learn how device manufacturers can benefit from this innovative approach.
ANVISA publishes final version (v1.3) of the Medical Device Registration Manual – January 2026
ANVISA publishes final version (v1.3) of its Medical Device Registration Manual. See what manufacturers need to know for regulatory submissions in Brazil.
FDA Updates Guidance on Clinical Decision Support (CDS) Software for Healthcare Professionals
New FDA guidance clarifies which Clinical Decision Support software functions are not considered medical devices. Key updates affect software developers and manufacturers.
New FDA Guidance Clarifies Policy for Low-Risk General Wellness Devices
The FDA has updated its guidance on low-risk general wellness devices, clarifying criteria for software, wearables, and lifestyle technologies that may fall outside device regulation. Effective January 6, 2026.
Swissmedic Publishes New Guidance on Scientific GMDP Meetings for Establishment Licence Holders
New Swissmedic guidance formalises the process for Scientific GMDP Meetings with Swiss establishment licence holders. Effective January 2026. Download the official document here.
Kickstart your 2026 Regulatory Strategy with Smart MDR
Did you miss our holiday announcement? Don't worry—there is still time to accelerate your market entry in 2026 with an exclusive advantage. Regulatory compliance shouldn't be a barrier to innovation. At Smart MDR, we are extending our special support for Startups to ensure your medical device, IVD, or SaMD hits the European market faster and more efficiently.
Comece 2026 com o pé direito no Mercado Europeu!
Natal passou, mas a sua oportunidade de levar inovação médica para a Europa com condições exclusivas ainda está de pé! Sabemos que os desafios regulatórios são a maior barreira para Startups de dispositivos médicos, IVDs e softwares (SaMD). Por isso, a Smart MDR decidiu manter nossa condição especial de início de ano para ajudar você a conquistar a Marcação CE em 2026.