EUDAMED Production v2.22.0: What Manufacturers Need to Know
EUDAMED Production v2.22.0 introduces EMDN versioning, improved traceability, and DTX updates. Learn what this means for manufacturers.
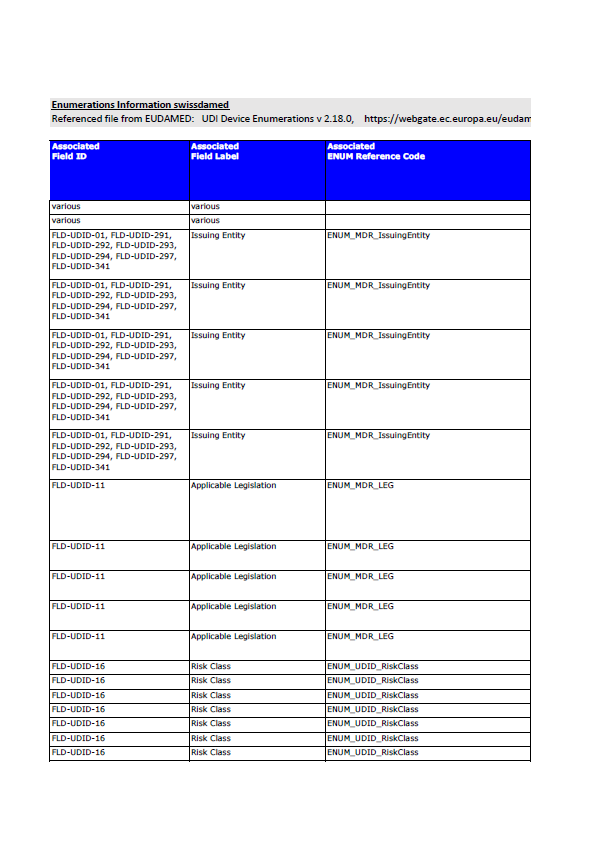
Swissmedic Publishes Updated EUDAMED UDI Enumerations: What Manufacturers Should Know
Swissmedic publishes updated EUDAMED UDI enumerations (v2.18.0). What this means for manufacturers preparing UDI, device and certificate data submissions.
Swissmedic Updates swissdamed Business Rules – Key Changes for Device Manufacturers in 2026
Swissmedic has released version 3.0 of its swissdamed Business Rules, effective January 2026. The update includes stricter UDI-DI requirements, limits on legacy device data, and mandatory fields for MDR/IVDR submissions. Manufacturers must now align with Swiss-specific data rules diverging from EUDAMED.
EUDAMED v2.18.0: Nova Atualização Traz Melhorias Importantes para Fabricantes e Distribuidores de Dispositivos Médicos
A EUDAMED é a base de dados europeia que centraliza informação sobre dispositivos médicos e de diagnóstico in vitro (IVD), essencial para a conformidade com o Regulamento (UE) 2017/745 (MDR) e o Regulamento (UE) 2017/746 (IVDR).
Com a atualização v2.18.0, fabricantes, distribuidores e representantes autorizados beneficiam de um sistema mais robusto, transparente e interoperável.
Nova regulamentação da Comissão Europeia simplifica atribuição de UDI a armações, lentes oftálmicas e óculos de leitura pré-montados
A Comissão Europeia adotou o Regulamento Delegado (UE) 2025/1920, introduzindo o conceito de Master UDI-DI para armações de óculos, lentes de óculos e óculos de leitura prontos a usar. Esta alteração reduz a proliferação de registos UDI na EUDAMED, simplifica a conformidade para os fabricantes e mantém a segurança dos pacientes — com aplicação a partir de 1 de novembro de 2028, embora seja permitida a adoção antecipada.
MDCG Clarifica Prazos para a Implementação do Master UDI-DI em Lentes de Contacto, Armações e Óculos de Leitura
O guia MDCG 2025-7 define os prazos e critérios para a implementação do Master UDI-DI em lentes de contacto, óculos de leitura, lentes oftálmicas e armações. A clarificação introduz datas de obrigatoriedade faseada, com destaque para 2026 e 2028, e orienta os fabricantes de dispositivos médicos a prepararem as suas estratégias de rastreabilidade e registo na EUDAMED.
Instruções de Utilização em Formato Eletrónico: Comissão Europeia Alarga o Âmbito do Regulamento
O novo Regulamento de Execução (UE) 2025/1234 alarga o âmbito das e-IFU para dispositivos médicos e acessórios utilizados por profissionais, incluindo dispositivos sem finalidade médica do Anexo XVI do MDR. A alteração reflete a preferência crescente por formatos digitais e exige que os fabricantes garantam acesso contínuo, rastreabilidade e conformidade técnica das IFU online.
EUDAMED: Updated Guide to Legacy Device Registration Now Available
The new EUDAMED Guide to the Registration of Legacy Medical Devices provides detailed and practical instructions for manufacturers operating under the MDD or AIMDD directives. The transition to MDR requires legacy devices to be identified with EUDAMED DI and EUDAMED ID, even in the absence of a UDI-DI. This registration in the EUDAMED database is essential to ensure regulatory compliance and maintain the placement of devices on the European market.
New version of the MIR form (v7.3.1) published on June 4 reinforces alignment with IMDRF coding
Version 7.3.1 of the MIR form, published by the European Commission on June 4, 2025, introduces new requirements for reporting serious incidents involving medical devices and IVDs.
EUDAMED version 2.15.0: New features and corrections to the European database of medical devices
A atualização da EUDAMED versão 2.15.0 introduz melhorias relevantes para o registo de dispositivos médicos e IVD, incluindo a gestão de UDI-DI, funcionalidades para Master UDI-DI em lentes de contacto, e novas opções de pesquisa no portal público. Esta versão corrige mais de 50 erros reportados pelos utilizadores, reforçando a fiabilidade da base de dados europeia de dispositivos médicos. Para os fabricantes, mandatários e importadores, é essencial rever os dados submetidos e preparar os processos internos para assegurar a conformidade com o MDR e IVDR.
MedTech Europe Publishes Practical Guide for Using the European Medical Device Nomenclature (EMDN)
MedTech Europe 's new practical guide to the European Medical Device Nomenclature (EMDN) provides key guidance for medical device manufacturers and IVDs. The correct assignment of EMDN codes is mandatory for registering devices with EUDAMED and for demonstrating compliance with the MDR and IVDR.
European Commission publishes new version of MIR form (v7.3.1)
The new version of the MIR 7.3.1 form, published by the European Commission, brings relevant changes for manufacturers of medical and in vitro diagnostic devices. The updated form is mandatory in the context of Regulation (EU) 2017/745 (MDR) and Regulation (EU) 2017/746 (IVDR), and is aligned with post-market surveillance (PMS) requirements and the future use of the EUDAMED system. Manufacturers must ensure that they use the new MIR model, with updated fields, IMDRF coding, EMDN nomenclature and structured data such as UDI-DI, Basic UDI and SRN.
EUDAMED User Guide for Legacy Devices - Essential Tool for Compliance with European Regulations
The new EUDAMED guide for legacy devices is an essential tool for medical device manufacturers who continue to operate under the previous MDD and AIMDD directives, but want to remain compliant during the transition period to Regulation (EU) 2017/745 (MDR). The document covers the assignment of EUDAMED DI and EUDAMED ID codes, the structured registration of legacy devices, the management of certificates and linking to already registered regulated devices. With practical guidelines and examples of accepted formats, this guide contributes to strengthening the traceability, compliance and regulatory security of legacy devices still on the European market.
New Version of the EUDAMED User Guide for Devices with UDI - An Essential Resource for Manufacturers
The new EUDAMED user guide for Unique Identifier (UDI) devices is an indispensable reference for all medical device manufacturers who want to ensure compliance with the MDR and IVDR regulations. The document covers aspects such as registering Basic UDI-DI and UDI-DI, managing data in EUDAMED, connecting to legacy devices, and updating versions. It also includes crucial information on requirements for accessing the EUDAMED platform, user profiles, and the procedures for structured data submission. This guide is essential for ensuring an effective compliance strategy in the European regulatory context.
MDCG 2025-1: Procedure Form for Updating the European Medical Device Nomenclature (EMDN)
MDCG 2025-1 introduces an ad hoc procedure form to facilitate the updating of the European Medical Device Nomenclature (EMDN). This document is crucial for manufacturers, national competent authorities (NCAs) and notified bodies (NBs) that need to register devices in the UDI-DI module of EUDAMED. The new approach allows proposals for new codes to be submitted when existing ones are insufficient, promoting the registration of innovative technologies and regulatory compliance. This initiative by the Medical Device Coordination Group (MDCG) contributes to standardisation and clarity in the medical device sector in the European Union.