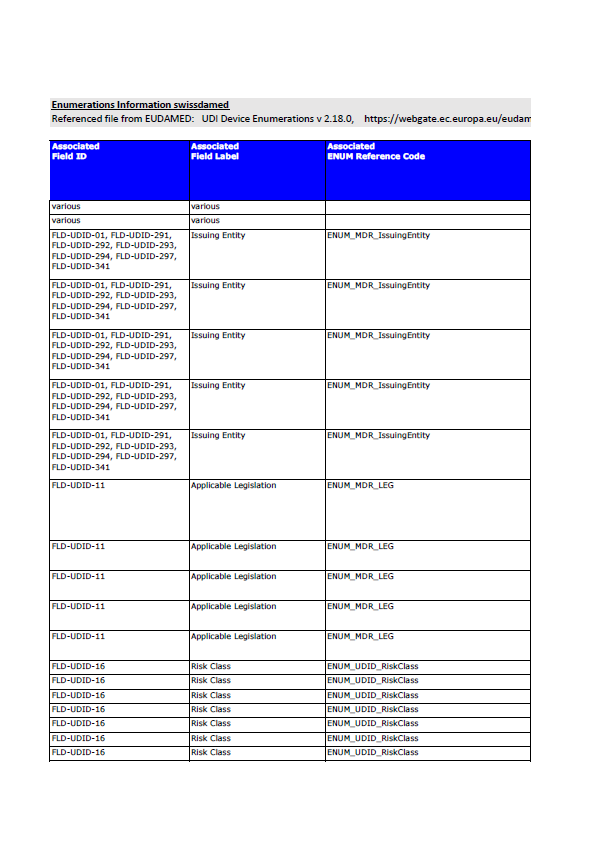
Swissmedic Publishes Updated EUDAMED UDI Enumerations: What Manufacturers Should Know
Swissmedic publishes updated EUDAMED UDI enumerations (v2.18.0). What this means for manufacturers preparing UDI, device and certificate data submissions.
Swissmedic Updates eIAM Portal Administrator Guidance – Version 3.4 Effective February 2026
Swissmedic publishes eIAM Portal Administrator guidance version 3.4, effective February 2026. Learn what this means for manufacturers using Swiss eGov services.
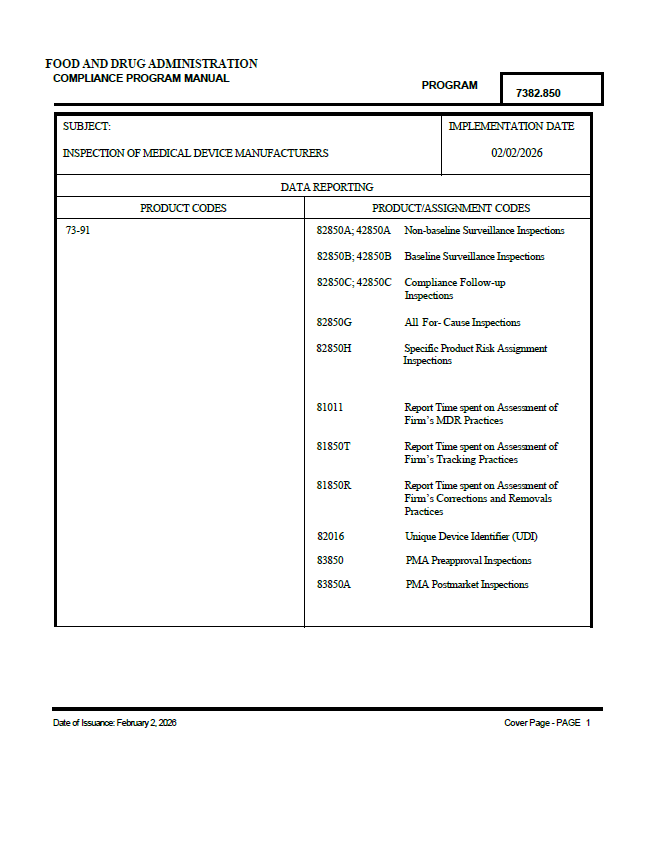
FDA Implements Updated Medical Device Inspection Compliance Program
FDA implements Compliance Program 7382.850, aligning medical device inspections with the Quality Management System Regulation and ISO 13485.
European Commission Launches the Digital Fitness Check: Implications for Medical Device Manufacturers
The European Commission has launched a Call for Evidence for its Digital Fitness Check, a major evaluation of EU digital legislation and its impact on competitiveness, innovation and administrative burden. This review is particularly relevant for medical device manufacturers facing overlapping digital, cybersecurity and data compliance obligations across EU rules.
EUDAMED: Updated Guide to Legacy Device Registration Now Available
The new EUDAMED Guide to the Registration of Legacy Medical Devices provides detailed and practical instructions for manufacturers operating under the MDD or AIMDD directives. The transition to MDR requires legacy devices to be identified with EUDAMED DI and EUDAMED ID, even in the absence of a UDI-DI. This registration in the EUDAMED database is essential to ensure regulatory compliance and maintain the placement of devices on the European market.
Coverage of MDR/IVDR Codes by Notified Bodies: June 2025 report highlights critical areas with low designation
The June 2025 report on the coverage of MDR and IVDR designation codes by notified bodies provides an updated view on the availability of designated bodies for specific categories of medical devices and in vitro diagnostic medical devices. It identifies critical areas with lower coverage, such as active implantable medical devices (codes MDA 0101 to MDA 0104) and devices originating from human tissues (MDS 1002), as well as IVDs for blood and tissue typing (codes IVR 0101 to IVR 0202). For medical device manufacturers seeking certification under Regulation (EU) 2017/745 or Regulation (EU) 2017/746, analysis of these codes is essential to plan the submission of applications to notified bodies with adequate coverage.
European Commission publishes new version of MIR form (v7.3.1)
The new version of the MIR 7.3.1 form, published by the European Commission, brings relevant changes for manufacturers of medical and in vitro diagnostic devices. The updated form is mandatory in the context of Regulation (EU) 2017/745 (MDR) and Regulation (EU) 2017/746 (IVDR), and is aligned with post-market surveillance (PMS) requirements and the future use of the EUDAMED system. Manufacturers must ensure that they use the new MIR model, with updated fields, IMDRF coding, EMDN nomenclature and structured data such as UDI-DI, Basic UDI and SRN.
EUDAMED User Guide for Legacy Devices - Essential Tool for Compliance with European Regulations
The new EUDAMED guide for legacy devices is an essential tool for medical device manufacturers who continue to operate under the previous MDD and AIMDD directives, but want to remain compliant during the transition period to Regulation (EU) 2017/745 (MDR). The document covers the assignment of EUDAMED DI and EUDAMED ID codes, the structured registration of legacy devices, the management of certificates and linking to already registered regulated devices. With practical guidelines and examples of accepted formats, this guide contributes to strengthening the traceability, compliance and regulatory security of legacy devices still on the European market.
New Version of the EUDAMED User Guide for Devices with UDI - An Essential Resource for Manufacturers
The new EUDAMED user guide for Unique Identifier (UDI) devices is an indispensable reference for all medical device manufacturers who want to ensure compliance with the MDR and IVDR regulations. The document covers aspects such as registering Basic UDI-DI and UDI-DI, managing data in EUDAMED, connecting to legacy devices, and updating versions. It also includes crucial information on requirements for accessing the EUDAMED platform, user profiles, and the procedures for structured data submission. This guide is essential for ensuring an effective compliance strategy in the European regulatory context.
Now you can talk to Smart MDR via WhatsApp
Smart MDR offers support via WhatsApp for manufacturers of medical devices, IVDs and medical device software looking for support with CE Marking and European regulations. Talk to an expert consultant and accelerate access to the European market with confidence.