Swissmedic Publishes Updated EUDAMED UDI Enumerations: What Manufacturers Should Know
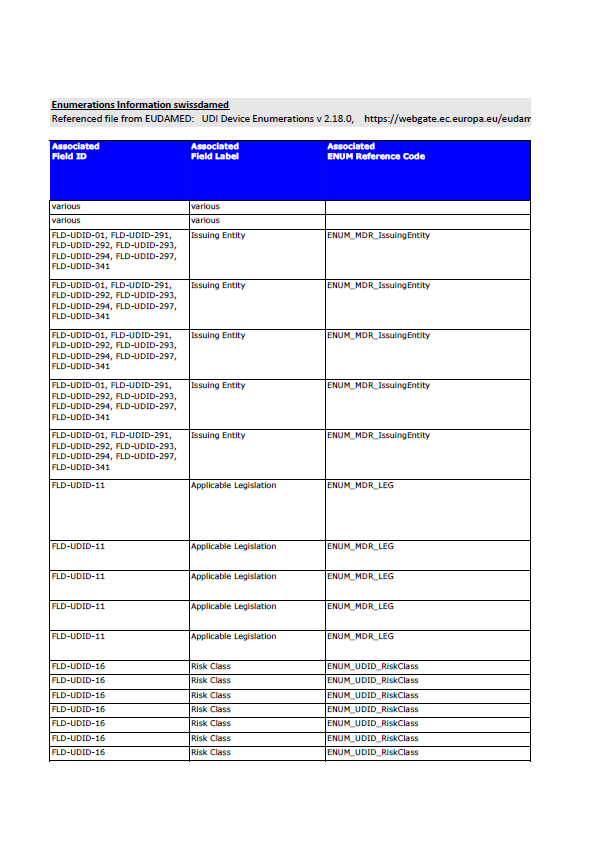
Swissmedic has published updated information referencing EUDAMED UDI Device Enumerations version 2.18.0, as defined in the European Commission’s EUDAMED technical documentation. The document consolidates the controlled enumeration values used across EUDAMED modules, with direct relevance for manufacturers responsible for UDI, device, and certificate data submissions.
This publication serves as a technical reference for the structured data fields required in EUDAMED and reflects the controlled vocabularies that manufacturers must use when registering devices, certificates, and related information.
What the document covers
The Swissmedic document compiles enumeration codes linked to specific EUDAMED field IDs. These enumerations are used in multiple regulatory data areas, including:
UDI issuing entities recognised under EU legislation
Applicable legislation identifiers, covering MDR, IVDR and legacy directives
Risk classes for medical devices and IVDs
Device and special device types, including software, systems, procedure packs, and Annex XVI products
Certificate types aligned with MDR, IVDR, MDD, AIMDD and IVDD
Clinical size descriptors and measurement units
Storage and handling conditions, critical warnings, and symbol-related codes
All values listed are controlled entries, meaning manufacturers must select from predefined options rather than use free text.
Why this matters for manufacturers
Although the document does not introduce new MDR or IVDR requirements, it is directly relevant to manufacturers preparing or maintaining EUDAMED registrations.
For manufacturers, the key impact is practical rather than legal:
Incorrect enumeration selection can lead to EUDAMED validation errors
Misalignment between EUDAMED data and technical documentation may delay submissions
UDI, regulatory, and IT systems must be aligned with the same enumeration values
As EUDAMED relies on structured data, manufacturers should ensure that internal systems (such as UDI databases, ERP or PLM tools) reflect the current EUDAMED-controlled vocabularies referenced in this document.
No regulatory change, but increased implementation clarity
The Swissmedic publication does not modify legal obligations under the MDR or IVDR. Instead, it provides implementation clarity by consolidating the enumeration values expected by EUDAMED. This is particularly relevant as manufacturers increase their readiness activities and transition from planning to active data submission.
Manufacturers are therefore encouraged to review this document as a reference tool to support accurate and consistent EUDAMED data entry.
Read the full document below.