New MHRA Summary: UK Conformity Assessment Routes for All Device Classes
The MHRA has published a helpful new summary outlining the UK MDR 2002 conformity assessment routes for medical devices, including Class I, Is/Im, IIa, IIb, III, AIMDs, systems/procedure packs, and custom-made devices. This visual guide is especially relevant for manufacturers placing products on the Great Britain market under the UKCA mark, and it provides a clear breakdown of the applicable annexes, UKRP obligations, and registration requirements.
Key Takeaways for Manufacturers
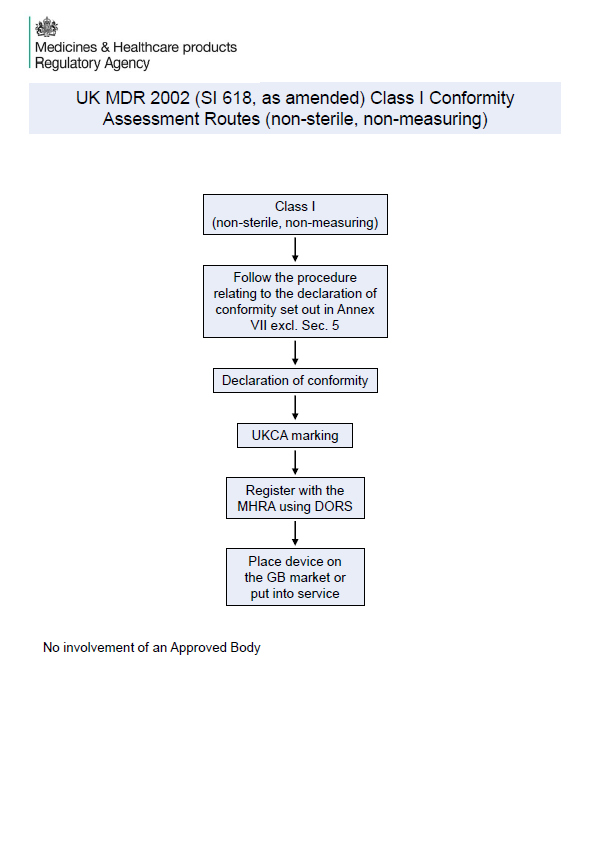
✅Class I (non-sterile, non-measuring): No Approved Body involvement. Manufacturers must follow Annex VII (excluding Section 5) and register with the MHRA via DORS. Devices must bear the UKCA mark before being placed on the GB market.
✅Class Is and Im (sterile or measuring): Involvement of an Approved Body is limited to the aspects of sterility or metrological requirements. Conformity routes include Annex II (excluding Section 4), IV, V, VI, and VII including Section 5.
✅Class IIa and IIb: Multiple conformity routes are available, including Annexes II, III, IV, V, VI, and VII, depending on device class and manufacturer strategy. Each requires UKCA marking and MHRA registration.
✅Class III and AIMDs: High-risk devices require Approved Body involvement. Manufacturers must follow Annex II (including Section 4), III, IV, or V, depending on their quality assurance system.
✅ Custom-Made Devices: These must not bear a UKCA mark but must be registered via DORS and be accompanied by a statement under Annex VIII or Annex 6, depending on device type (general or active implantable). Manufacturers or their UKRP are responsible for issuing the statement.
✅Systems & Procedure Packs: If sterilisation is involved, manufacturers must follow Annex IV or II (limited to sterility aspects). A declaration under Regulation 14 applies, and all components must already have valid declarations of conformity.
This summary reinforces the existing structure under the UK MDR 2002 and provides practical reference points for conformity strategy planning, especially as we approach the transition deadlines for full UKCA compliance.
Access the full MHRA document below.