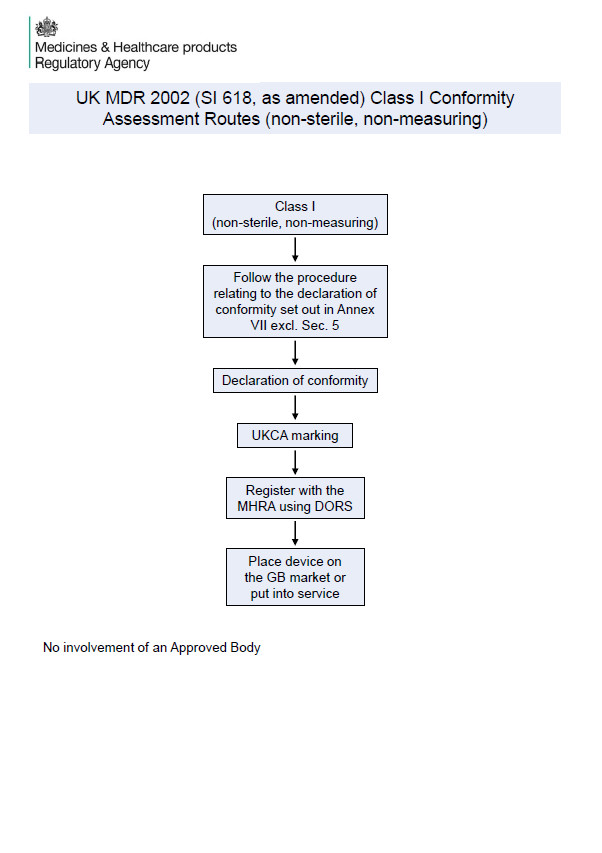
New MHRA Summary: UK Conformity Assessment Routes for All Device Classes
New MHRA guidance summarises UK conformity routes for all medical device classes. Essential for UKCA compliance and UK MDR planning.
Australia Updates Guidance on Export Certificates for Medical Devices: What EU Manufacturers Need to Know
The TGA has updated its guidance on Certificates of Free Sale and Export Certificates for medical devices. Key changes for EU manufacturers exporting via Australia.