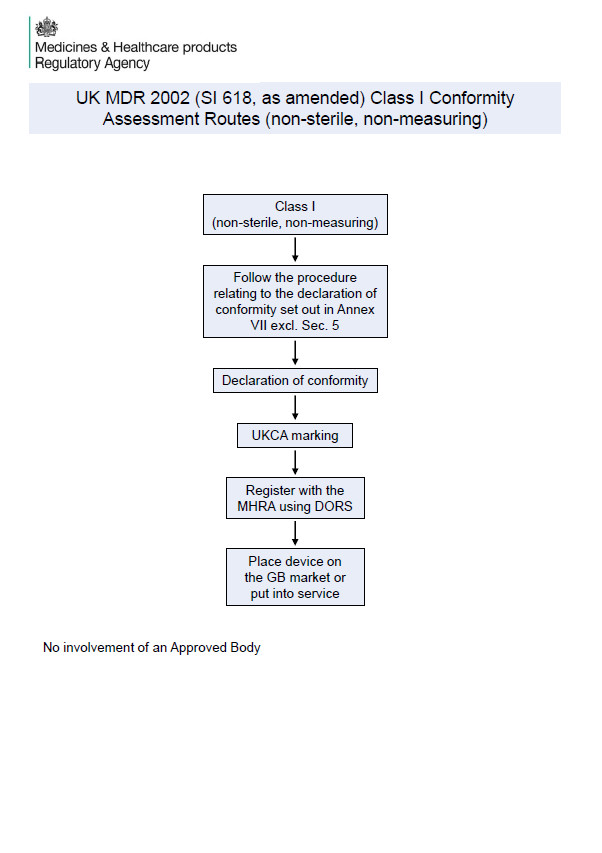
New MHRA Summary: UK Conformity Assessment Routes for All Device Classes
New MHRA guidance summarises UK conformity routes for all medical device classes. Essential for UKCA compliance and UK MDR planning.
MHRA Updates Guidance on Clinical Investigations – What Manufacturers Need to Know
The MHRA has released updated guidance for clinical investigations of medical devices in the UK, outlining new requirements for manufacturers, including application validation, safety reporting, UKCA/CE/CE UKNI considerations, and updated processes for studies in Great Britain and Northern Ireland. Medical device manufacturers must review these regulatory changes to maintain compliance, ensure timely clinical investigation approvals, and prepare robust technical documentation aligned with UK and EU regulatory frameworks.