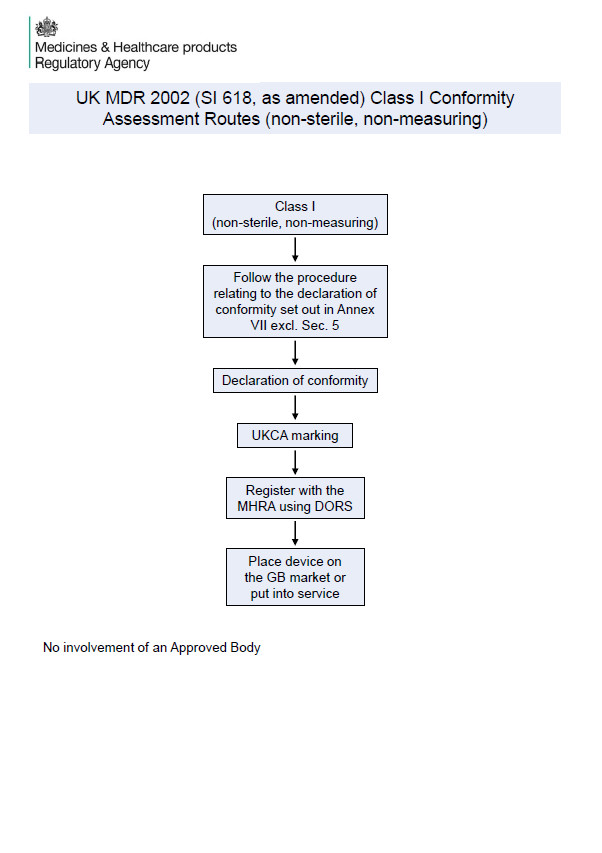
New MHRA Summary: UK Conformity Assessment Routes for All Device Classes
New MHRA guidance summarises UK conformity routes for all medical device classes. Essential for UKCA compliance and UK MDR planning.
Swissmedic Highlights Critical Deficiencies in Notified Body Opinions for Integral Combination Products
Swissmedic’s review of Notified Body Opinions under MDR Article 117 reveals major gaps in documentation quality for drug–device combination products. Updated expectations now apply to ensure compliance with GSPRs.
Team-NB's New Code of Conduct: Towards a More Harmonized and Transparent Evaluation
The new version of the Team-NB Code of Conduct establishes principles of transparency, coherence and quality in the work of Notified Bodies operating under the MDR and IVDR regulations. The document presents clear criteria for audits, technical review, management of conflicts of interest, use of external experts and deadlines for responding to non-conformities.
MDCG publishes guide on the joint application of the MDR, IVDR and AI Act: what manufacturers should know
The new MDCG 2025-6 guide, published in June 2025, clarifies the joint application of the AI Act and the European MDR/IVDR regulations for medical devices and IVDs with artificial intelligence systems. The document addresses classification, risk management, life cycle, technical documentation and post-market monitoring. Smart MDR supports manufacturers in integrating these requirements into their quality management system and preparing for audits.
Team NB Publishes New Good Practice Guide for the Submission of Technical Documentation under the MDR
The new version of Team NB's good practice guide for submitting technical documentation under Regulation (EU) 2017/745 (MDR) provides clear guidance for medical device manufacturers. The document addresses the structuring of dossiers, the consistency of data, common mistakes to avoid and the importance of effective communication with Notified Bodies.
European Commission Publishes New MDR Harmonized Standards: Direct Impact on Medical Device Compliance
Implementing Decision (EU) 2025/681 introduces six new harmonized standards under the Medical Devices Regulation (MDR), directly impacting manufacturers of single-use medical gloves, sterile devices and ambulance patient transport equipment. Compliance with these standards allows presumption of conformity with the essential requirements of the MDR, facilitating CE marking and access to the European market. Smart MDR supports manufacturers in reviewing technical documentation, assessing regulatory impact and adapting processes to the new European legislation.
MDCG 2019-6 Rev5 | MDCG Update on Requirements for Notified Bodies
Revision 5 of MDCG 2019-6 presents important updates on the requirements for Notified Bodies under the MDR and IVDR, reinforcing regulatory compliance and promoting harmonisation within the European Union. It includes clarifications on impartiality and independence, as well as specifications on consultancy, training and experience of personnel involved in conformity assessment.