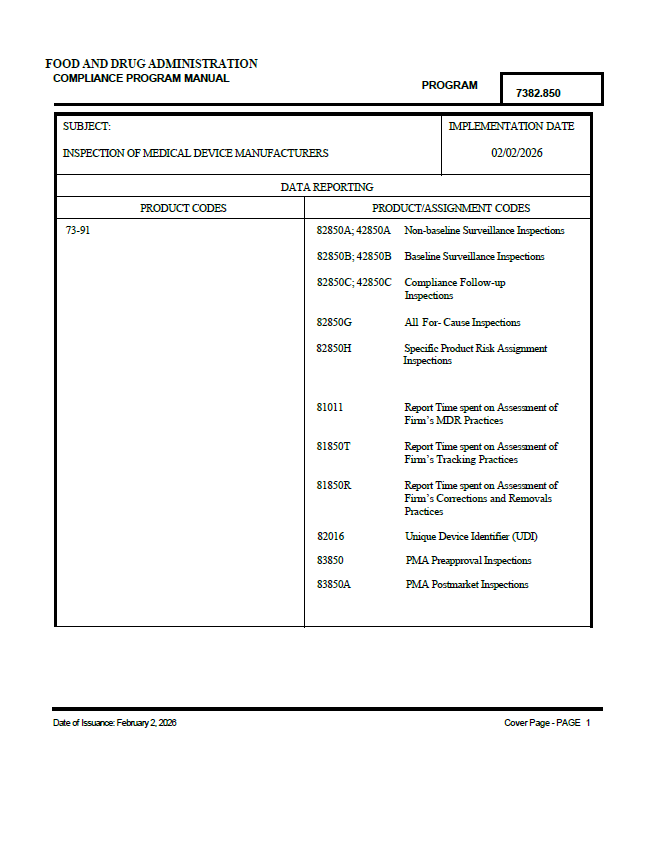
FDA Implements Updated Medical Device Inspection Compliance Program
FDA implements Compliance Program 7382.850, aligning medical device inspections with the Quality Management System Regulation and ISO 13485.
FDA Announces Expanded Use of Unannounced Inspections at Foreign Manufacturing Facilities
The FDA 's recent decision to expand the use of unannounced inspections at manufacturing facilities outside the United States has relevant implications for medical device and drug manufacturers exporting to the US market. This approach requires manufacturers to maintain robust quality systems, permanently updated documentation and a culture of continuous compliance.